Abstract
Background: Standard therapies for acute myeloid leukemia (AML) include intensive chemotherapy and low-intensity regimens selected based on patient age and/or comorbidities. Both are effective in inducing deep and durable responses. Measurable residual disease (MRD) is a strong, independent prognostic factor for predicting overall survival (OS) and relapse in AML. We hypothesized that patients (pts) achieving MRD negativity may experience similar outcomes irrespective of the treatment regimen applied to achieve the response.
Methods: In this retrospective chart review study, we selected all newly-diagnosed AML pts treated at our institution between 2010 and 2021 who had achieved a first response (CR, CRi, or MLFS) and had undergone MRD testing by flow cytometry at the time of best response. Pts with core-binding factor AML and acute promyelocytic leukemia, as well as pts who had received prior therapy for antecedent myelodysplastic syndrome, were excluded. We divided pts into 2 cohorts: cohort IA consisting of pts receiving intensive chemotherapy based on intermediate to high-dose cytarabine plus an anthracycline (without venetoclax), and cohort LO + VEN consisting of pts treated with a low-dose cytarabine or hypomethylating agent backbone plus venetoclax. The Kaplan Meier method was used to calculate OS and time-to-relapse (TTR). Pts who underwent stem cell transplantation (SCT) were censored at the time of transplant. Multivariate analysis was performed using a Cox proportional hazards model.
Results: We identified 635 pts meeting inclusion criteria (385 IA, 250 LO + VEN). Compared to pts treated with IA, pts treated with LO + VEN were significantly older (median age 71.6 vs 52.8 yrs), had a lower rate of true CR (76% vs 89%), had a higher rate of CRi/MLFS (24% vs 11%), were less likely to achieve MRD-negativity (60% MRD(-) vs 71%), and were less likely to have undergone SCT (25% vs 50%). In addition, the LO + VEN cohort was enriched for ELN 2017 adverse risk disease (58% vs 34% in the IA cohort) and adverse risk cytogenetic features such as -5/5q- (19% vs 7%), -7/7q- (17% vs 7%), -17/17p- (12% vs 5%), and complex karyotype (29% vs 16%).
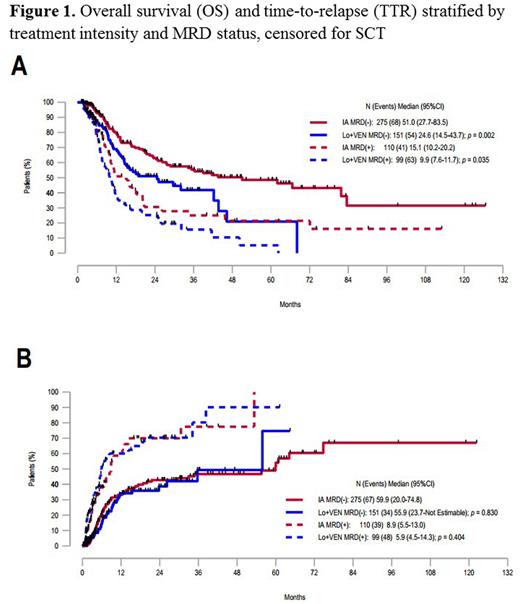
The median OS was 51m, 24.6m, 15.1m, and 9.9m in the IA MRD(-), LO + VEN MRD(-), IA MRD(+), and LO + VEN MRD(+) cohorts, respectively (fig. 1A). The median TTR was 59.9m, 55.9m, 8.9m, and 5.9m in the IA MRD(-), LO + VEN MRD(-), IA MRD(+), and LO + VEN MRD(+) cohorts, respectively (fig. 1B). When comparing the IA MRD(-) cohort to the LO + VEN MRD(-) cohort, the IA-treated patients had superior OS (p=0.002, fig. 1A) but similar TTR (p=0.830, fig. 1B). Similar findings were obtained in the MRD(+) pairs (fig. 1A-B). A subset analysis evaluating only pts aged ≥ 60 yrs (n=297) showed no statistically significant differences in OS or TTR within MRD categories (median OS 28.9m in IA MRD(-) vs 24.6m in LO + VEN MRD(-), p=0.09; median OS 15.1m in IA MRD(+) vs 10.6m in LO + VEN MRD(+), p=0.51; median TTR not reached in IA MRD(-) vs 35.7m in LO + VEN MRD(-), p=0.32; median TTR 9.6m in IA MRD(+) vs 6.0m in LO + VEN MRD(+), p=0.43).
Given the imbalances in patient and disease characteristics between the treatment cohorts, we performed a multivariate analysis on the full cohort to better elucidate the relative prognostic impact of MRD and treatment intensity. By univariate analysis, age, treatment cohort (IA vs LO + VEN), best response (CR/CRi/MLFS), MRD status, and ELN risk were significant predictors for OS and were included in the multivariate model. By multivariate analysis, age (HR 1.02, p=0.026), best response (HR 1.41 for CRi, p=0.066; HR 2.69 for MLFS, p<0.001), MRD-status (HR 1.53 for MRD(+), p=0.007), and ELN category (HR 1.34 for intermediate, p=0.161, HR 1.96 for adverse, p=0.001) remained significant predictors of OS. For TTR, best response (HR 2.15 for CRi, p<0.001; HR 3.78 for MLFS, p<0.001), MRD-status (HR 1.94 for MRD(+), p<0.001), and ELN category (HR 1.79 for intermediate, p=0.014, HR 2.89 for adverse, p<0.001) remained significant. Treatment received was not predictive of either OS or TTR by multivariate analysis.
Conclusions: MRD status is a stronger predictor of OS and TTR than the intensity of therapy received. These data suggest achievement of MRD-negativity should be a major objective of AML therapy.
Disclosures
Kadia:PinotBio: Consultancy; cyclacel: Research Funding; Amgen: Research Funding; AstraZeneca: Research Funding; Astellas: Research Funding; cellenkos: Research Funding; Delta-Fly: Research Funding; Servier: Consultancy; Iterion: Research Funding; Genfleet: Research Funding; Pfizer: Research Funding; Novartis: Consultancy; Genentech: Consultancy, Research Funding; JAZZ: Consultancy, Research Funding; Glycomimetics: Research Funding; Astex: Honoraria; Regeneron: Research Funding; BMS: Consultancy, Research Funding; Ascentage: Research Funding; Agios: Consultancy; Abbvie: Consultancy, Research Funding. Short:Astellas: Research Funding; Pfizer: Consultancy; Novartis: Consultancy; Takeda Oncology: Consultancy, Research Funding; Amgen: Consultancy, Honoraria; Stemline Therapeutics: Research Funding; AstraZeneca: Consultancy. Borthakur:Pacylex, Novartis, Cytomx, Bio Ascend: Membership on an entity's Board of Directors or advisory committees; Catamaran Bio, Abbvie, PPD Development, Protagonist Therapeutics, Janssen: Consultancy; Astex Pharmaceuticals, Ryvu, PTC Therapeutics: Research Funding. Loghavi:Abbvie: Consultancy, Current equity holder in publicly-traded company; QualWorld: Consultancy; Amgen: Research Funding; Astellas: Research Funding; GLG: Consultancy; PeerView: Honoraria. DiNardo:Gilead: Honoraria; Bristol Myers Squibb: Honoraria, Research Funding; Servier: Consultancy, Honoraria, Research Funding; Cleave: Research Funding; Takeda: Honoraria; Bluebird Bio: Honoraria; Novartis: Honoraria; LOXO: Research Funding; ImmuneOnc: Honoraria, Research Funding; Kura: Honoraria, Membership on an entity's Board of Directors or advisory committees; Foghorn: Honoraria, Research Funding; Astex: Research Funding; Forma: Research Funding; GenMab: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Astellas: Honoraria; AbbVie: Consultancy, Research Funding; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Jazz: Honoraria. Daver:Agios, Celgene, SOBI and STAR Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kartos and Jazz Pharmaceuticals: Other: Data monitoring committee member; Karyopham Therapeutics and Newave Pharmaceutical: Research Funding; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Novartis, Jazz, Amgen, Servier, Karyopharm, Trovagene, Trillium, Syndax, Gilead, Pfizer, Bristol Myers Squibb, Kite, Actinium, Arog, Immunogen, Arcellx, and Shattuck: Consultancy, Other: Advisory Role; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Gilead, Immunogen, Pfizer, Bristol Myers Squibb, Trovagene, Servier, Novimmune, Incyte, Hanmi, Fate, Amgen, Kite, Novartis, Astex, KAHR, Shattuck, Sobi, Glycomimetics, Trillium: Research Funding. Alvarado:BerGenBio: Research Funding; Daiichi-Sankyo/Lilly: Research Funding; Jazz Pharmaceuticals: Research Funding; Astex Pharmaceuticals: Research Funding; FibroGen: Research Funding; Sun Pharma: Research Funding. Andreeff:Kintor Pharmaceutical: Research Funding; Breast Cancer Research Foundation: Research Funding; Daiichi-Sankyo Inc.: Consultancy, Research Funding; AstraZeneca: Research Funding; Syndax: Consultancy, Research Funding; Brooklyn ITX: Research Funding; Pinot Bio: Research Funding; Oxford Biomedical UK: Research Funding; Senti Bio: Consultancy, Research Funding; Aptose: Consultancy, Membership on an entity's Board of Directors or advisory committees; Glycomimetics: Consultancy; Medicxi: Consultancy; Cancer UK: Membership on an entity's Board of Directors or advisory committees; Leukemia & Lymphoma Society: Membership on an entity's Board of Directors or advisory committees; German Research Council: Membership on an entity's Board of Directors or advisory committees; NCI: Membership on an entity's Board of Directors or advisory committees; CLL Foundation: Membership on an entity's Board of Directors or advisory committees; Reata: Current holder of stock options in a privately-held company; Chimerix: Current holder of stock options in a privately-held company; Oncolyze: Current holder of stock options in a privately-held company. Jabbour:Spectrum: Research Funding; Takeda: Other: Advisory Role, Research Funding; Pfizer: Other: Advisory Role, Research Funding; Bristol Myers Squibb: Other: Advisory Role, Research Funding; Adaptive Biotechnologies: Other: Advisory Role, Research Funding; Amgen: Other: Advisory Role, Research Funding; Genentech: Other: Advisory Role, Research Funding; AbbVie: Other: Advisory Role, Research Funding. Konopleva:F. Hoffman La Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Grant support, Research Funding; AbbVie: Consultancy, Other: grant support, Research Funding; Genentech: Consultancy, Other: grant support, Research Funding; Stemline Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy; Forty-Seven: Consultancy, Honoraria, Other: Grant support; Kisoji: Consultancy, Honoraria; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Reata Pharmaceuticals: Current equity holder in private company, Patents & Royalties; Eli Lilly: Consultancy, Patents & Royalties, Research Funding; Cellectis: Consultancy, Other: Grant support, Research Funding; Calithera: Other: Grant Support, Research Funding; Ablynx: Other: Grant support, Research Funding; Agios: Other: grant support, Research Funding; Ascentage: Other: grant support, Research Funding; AstraZeneca: Other: grant support, Research Funding; Rafael Pharmaceutical: Other: grant support, Research Funding; Sanofi: Other: grant support, Research Funding; Novartis: Patents & Royalties, Research Funding. Kantarjian:Takeda: Honoraria; Jazz Pharmaceuticals: Research Funding; NOVA Research: Honoraria; KAHR Medical Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; Ipsen Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; ImmunoGen: Research Funding; Daiichi-Sankyo: Consultancy, Research Funding; Astellas Health: Honoraria, Membership on an entity's Board of Directors or advisory committees; Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding. Ravandi:BMS/Celgene: Consultancy, Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Prelude: Research Funding; Novartis: Consultancy; Abbvie: Consultancy, Honoraria, Research Funding; Biomea Fusion, Inc.: Research Funding; Syos: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy; Astellas: Consultancy, Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Xencor: Research Funding; Astex/Taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal